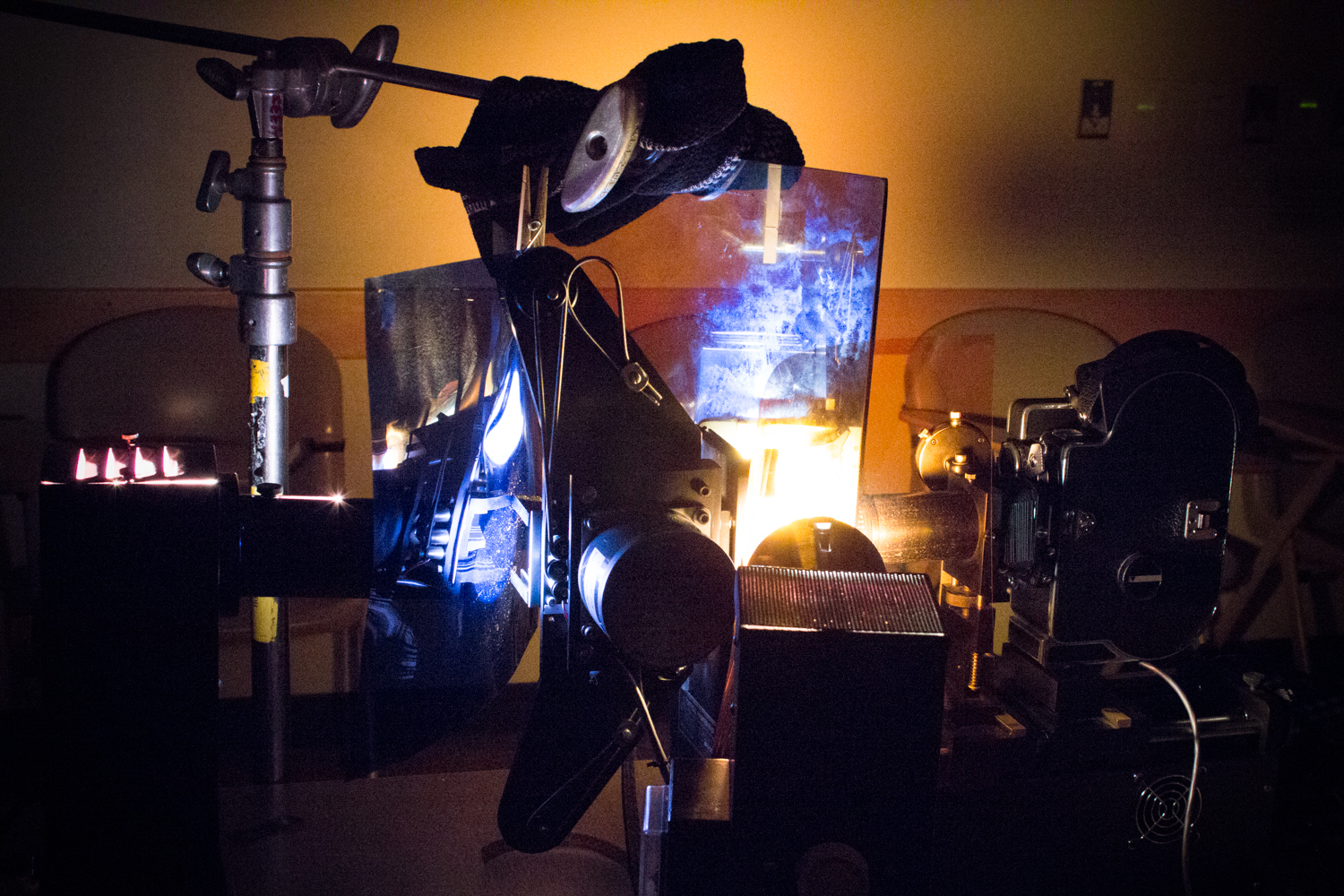HAPPY BELATED WORLD PINHOLE DAY!
On April 29 & April 30, 2017 Process Reversal and Redline Contemporary Art Center joined forces to celebrate Doors Open Denver and World Pinhole Day, welcoming over 100 Denverites of all ages to Redline for a weekend of free workshops and demos. Curt, Eric, Sarah and Laura showed Redline visitors how to shoot, process, and project their own 16mm pinhole movies while Denver-based artist and PR friend Eileen Richardson manned a 16mm direct animation station. Robert Schaller of the Handmade Film Institute joined us on day 2 to build and demonstrate his home-made pinhole device design, constructed out of daylight-boxes.

Robert Schaller of The Handmade Film Institute discussing Pinhole Filmmaking.

Modifying daylight boxes for a custom pinhole camera.
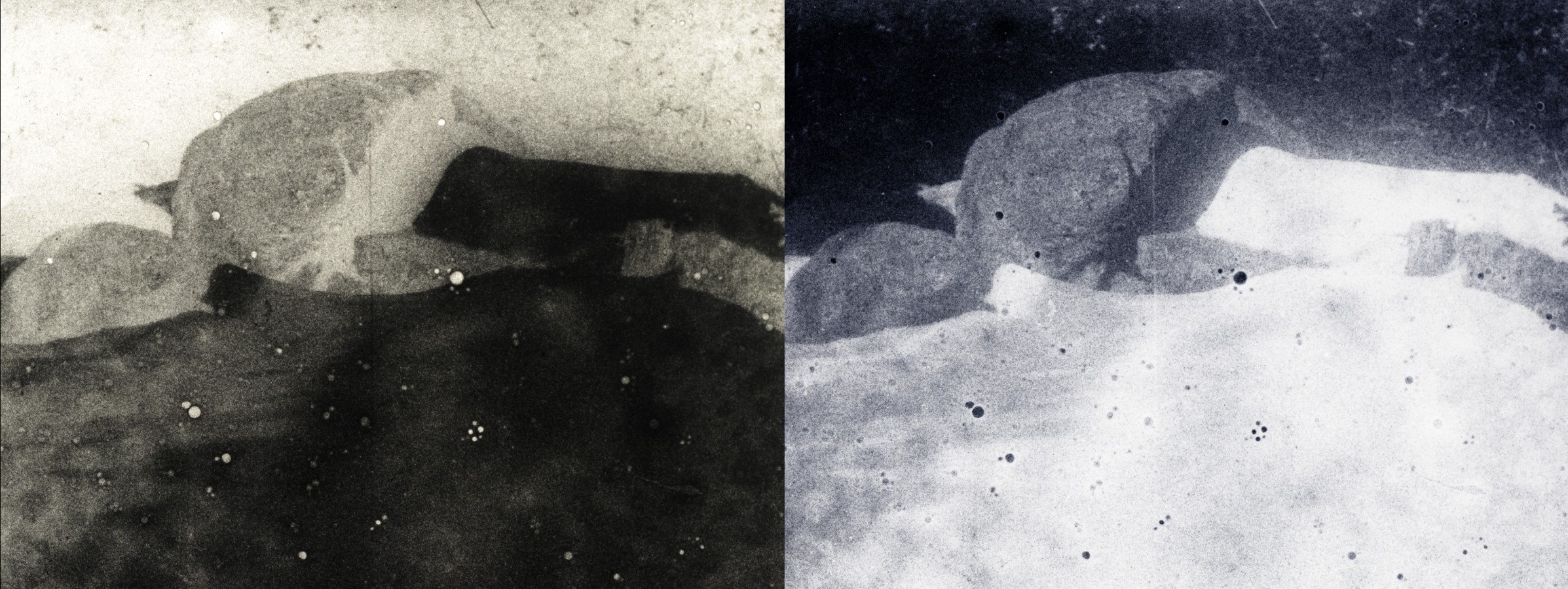
We used Bolex cameras with modified “lenses” of pinhole-punched aluminum pie-plates. The three cameras each had a slightly different sized pinhole, giving us three different f-stops and three different results in terms of the softness of the image. A fierce spring snow storm on Saturday forced us to shoot indoors with a light kit –we experimented with hand cranking at extremely slow frame rates or long single frame exposures in order to capture an image.

Shooting indoors, frame by frame.
Luckily Sunday cleared up and folks were able to shoot outdoors in the bright sunshine at 12 frames per second.
We shot on Tri-X, Double-X, and Hi-Con film stocks. In spite of intermittently snowy conditions and cloudy skies everyone produced some superb pinhole footage! Check it out below:

Happy World Pinhole Day 2017!